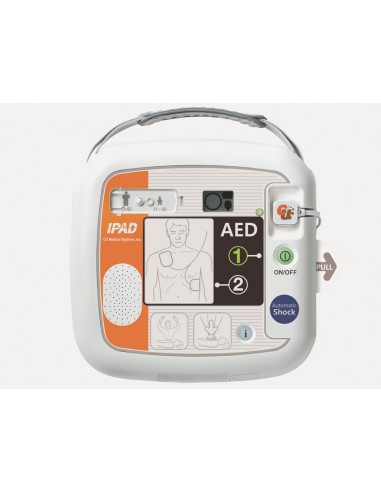
iPad CU-SP1 DEFIBRILLATOR - automatic specify language with order
• Operating mode: automatic
• Waveform: e-cube biphasic (Truncated exponential type)
• Energy: - 150J fixed - Adult: nominal 150J at 50 ohm load - Infant/child: nominal 50 joules at 50 ohm
• Charge control: controlled by automated patient analysis system
• Charging time: within 10 seconds since voice instruction "an electric shock is needed"
• Sealing: - waterjet proof IPX5 per IEC60529 (IP55) - dust protected IP5X per IEC60529 USER INTERFACE:
• User support: detailed voice prompts and flashing indicators
• CPR guidance: voice prompts for how to perform CPR for adult and child
• Controls: on/off button, ”i“ button, shock button
• Indicator: LCD display (Device status, Battery status, Pads status)
• Sensing: pads expiry date, Pads connection status
• CPR Monitoring
• Automatic Volume adjustment DATA RECORDING AND TRANSMISSION:
• IrDA port: wireless transmission of event data to PC, SD Card
• Internal Memory (Nand-flash): ECG, Event
• Storage capacity: multi recording 5 events / Max 3 hours
• Data review PC Programme: CU-EX1 PATIENT ANALYSIS SYSTEM:
• Patient Analysis: Shockable Rhythms - Ventricular Fibrillation, Tachycardia
• Sensitivity/Specificity Meets AAMI DF80 Guideline DISPOSABLE HIGH CAPACITY BATTERY:
• Standard: - Type: DC 12 Volt 4.2 Ah, Lithium manganese dioxide - Capacity: Minimum 200 shocks (150J)
• Auto volume adjustment: It detects the ambient noise and automatically adjusts the volume of its prompts to make them audible over the background noise.
• Smart pads - intelligent storage: The pre-connected "Smart" electrode pads are stored in an easy access compartment on the underside of the unit. This ensures that the iPAD SP1 is always ready to be used in the shortest time possible. In addition, the visual frontal indicator shows if the connected pads are within their life expectancy. The indicator will change when the pads have only 3 months life left before their expiry date giving you time to arrange for replacements. It will change again when the expiry date is reached.
• CPR detection: CPR ensures that the casualty has the best chances of survival. The CU-SP1 detects if CPR is being performed with appropriate prompts, if not it encourages the responder to perform it.
• Easy communication with CU-EX1 software: Installing CU-EX1 software onto a computer allows you to see and analyze the usage history of the unit. Information such as time of "power on", the casualty's heart rhythm and shocks delivered are all clearly presented.
• 16 languages available (to be indicated with order) Selectable software languages: US, GB, FR, IT, ES, DE, PT, NL, PL, SE, DK, NO, CZ, GR, AE, KR, IL. On request LT. The iPAD CU-SP1 can store up to 5 events with up to 3 hours of ECG analysis on an SD memory card. Supplied with adult SAVE PADS, carrying bag, disposable lithium battery pack, paper manual (GB, IT) and CD manual in above languages excluding PL and GR.
TECNICAL SPECIFICATIONS
DEFIBRILLATOR:
• Operating mode: automatic
• Waveform: e-cube biphasic (Truncated exponential type)
• Energy: - 150J fixed - Adult: nominal 150J at 50 ohm load - Infant/child: nominal 50 joules at 50 ohm
• Charge control: controlled by automated patient analysis system
• Charging time: within 10 seconds since voice instruction "an electric shock is needed"
• Sealing: - waterjet proof IPX5 per IEC60529 (IP55) - dust protected IP5X per IEC60529 USER INTERFACE:
• User support: detailed voice prompts and flashing indicators
• CPR guidance: voice prompts for how to perform CPR for adult and child
• Controls: on/off button, ”i“ button, shock button
• Indicator: LCD display (Device status, Battery status, Pads status)
• Sensing: pads expiry date, Pads connection status
• CPR Monitoring
• Automatic Volume adjustment DATA RECORDING AND TRANSMISSION:
• IrDA port: wireless transmission of event data to PC, SD Card
• Internal Memory (Nand-flash): ECG, Event
• Storage capacity: multi recording 5 events / Max 3 hours
• Data review PC Programme: CU-EX1 PATIENT ANALYSIS SYSTEM:
• Patient Analysis: Shockable Rhythms - Ventricular Fibrillation, Tachycardia
• Sensitivity/Specificity Meets AAMI DF80 Guideline DISPOSABLE HIGH CAPACITY BATTERY:
• Standard: - Type: DC 12 Volt 4.2 Ah, Lithium manganese dioxide - Capacity: Minimum 200 shocks (150J)
- Classe Medicale
- II B
- Dispositivo Medico
- Dispositivo medico
- unità
- 1 pz.
- GMDN
- 48047